Culture shift: tackling antimicrobial resistance from agriculture to operating table
Antibiotics have saved millions of lives since their discovery. Research from experts at The University of Manchester is helping to address this global crisis and provide better protection for us allAntibiotics have saved millions of lives since their discovery. However, globally increasing levels of antimicrobial resistance (AMR) are reducing the effectiveness of these crucial drugs. Research from experts at The University of Manchester is helping to address this global crisis and provide better protection for us all
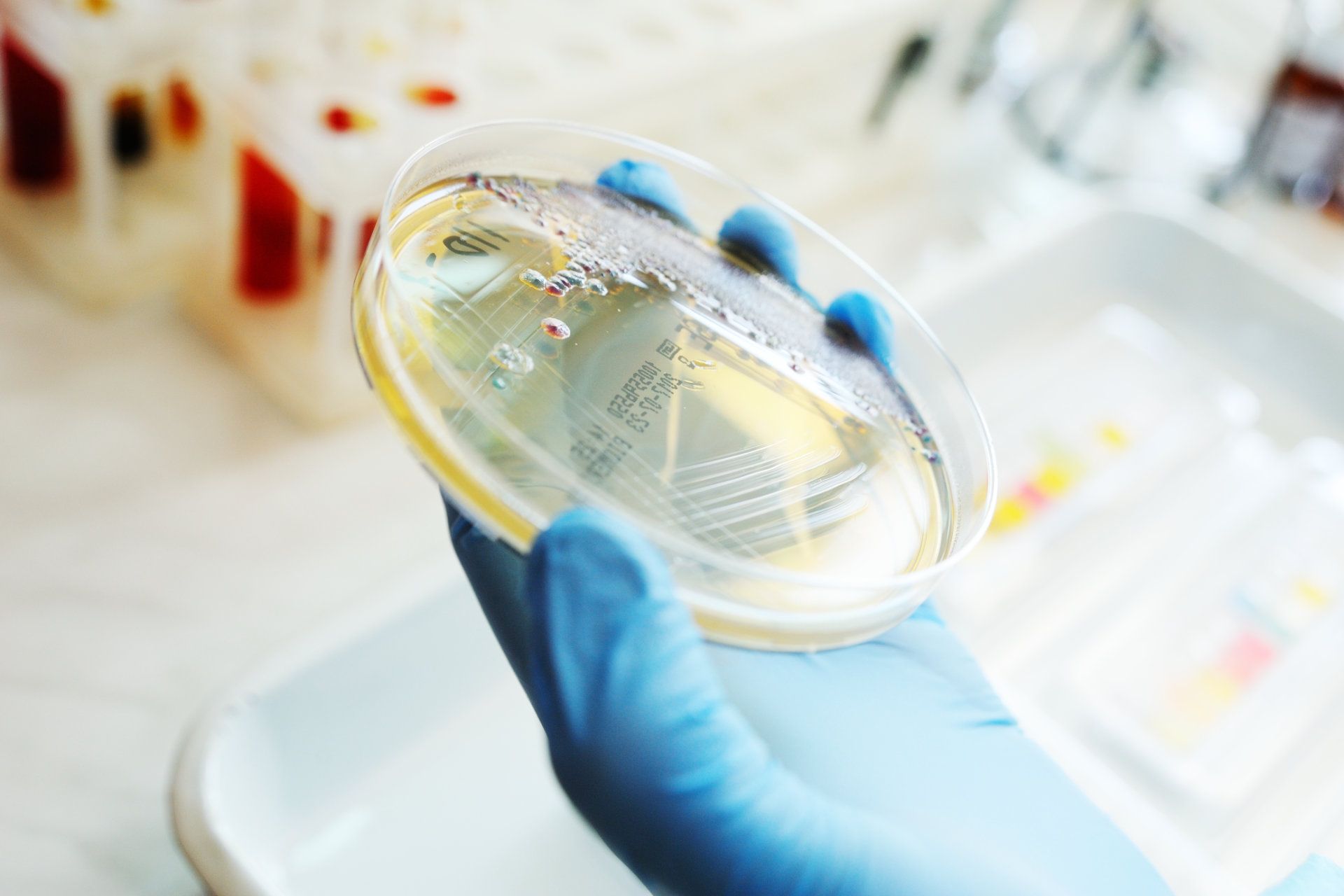
Antibiotics have been a key weapon in the fight against a range of infections and diseases since their discovery almost a century ago. We have all grown used to having easy access to these medicines to help us get better when we are ill. But what causes those medicines to stop working – and what happens if they do?
Globally, increasing levels of antimicrobial resistance (AMR) mean that these crucial drugs are no longer effective for treating many bacterial, viral, fungal, and protozoal infections such as malaria. Keeping antimicrobial drugs working has been highlighted as a global priority by the United Nations (UN) and World Health Organisation (WHO).
That challenge is something that Policy@Manchester, The University of Manchester’s policy engagement unit, has been seeking to address. The University’s AMR Network is working both to understand the root causes of AMR and to develop new solutions that could potentially save millions of lives.
A recent contribution to Policy@Manchester’s On Resilience publication details the various approaches researchers from across the University are taking to address AMR. In particular, the report considers the origins of AMR, the complexity of the relationships between clinical and agricultural uses, and identifies potential solutions that may help tackle this global crisis.